 |
| Jacqueline Smith, a Senior Research Associate at the Allen Institute for Cell Science, in the lab working on cell lines for the Allen Cell Collection. Photo by Erik Dinnel / Allen Institute |
A Q&A with Jacqueline Smith at the Allen Institute for Cell Science
The Coriell Institute for Medical Research has been the trusted biobank for organizations of all sizes for decades, and Coriell currently houses a special collection of healthy human induced pluripotent stem cells (hiPSCs) named the Allen Cell Collection. These hiPSC lines come from the Allen Institute for Cell Science and they were first made openly available to scientists in 2016. All cells in this collection have been fluorescently tagged through genome editing, enabling scientists to observe the inner workings of live cells with unprecedented clarity. This growing collection holds more than 50 lines, all of which illuminate key cellular structures and substructures at normal endogenous expression levels.
Jacqueline Smith, a Senior Research Associate at the Allen Institute, who manages cryopreservation for the vials and cell line quality control, answered a few questions about this state-of-the-art collection.
Tell me a little about the Allen Institute for Cell Science.
We want to know what healthy human cells look like and how they behave. We want to see and understand more about healthy cell organization and variation, and what goes wrong during disease. Better yet, we want to be able to make predictions about cell behaviors just by observing the cells at one moment in time. Our cell lines, plasmids, visualization tools, software, and education resources are all openly available for the scientific community to accelerate research and discovery.
What led your team to develop the Allen Cell Collection? What scientific need were you answering at the time?
The images of cells we all saw in our high school textbooks don’t accurately depict cells and their structures. Until recently, we didn’t have accurate visualizations of human cells. As you can imagine, it’s extremely difficult to study something that you can’t see very well. Our team developed the Allen Cell Collection with illuminated cellular structures and substructures so that we – and other scientists – could observe cells and study their behaviors with more clarity.
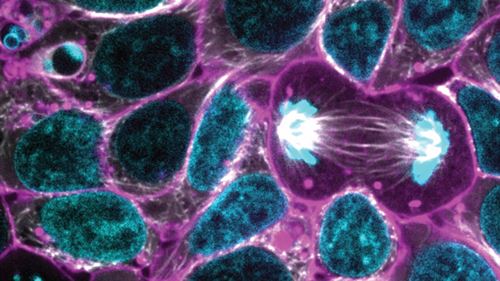 |
This image from the Allen Cell Collection shows hiPSCs illuminating alpha tubulin |
Can you talk a bit about the technology that goes into these lines?
Technology changes rapidly, so we’re changing with it. We are constantly pushing ourselves to streamline processes to minimize cell death and improve quality control. We have recently switched our CRISPR-Cas9 method of editing the donor into the genome, from plasmid-based to an adeno-associated virus (AAV) backbone. This new method of editing has shown significantly higher efficiency and we have recently released the first line in our collection, which tags CDH1, using this method. To determine correctly edited cells, we have improved our screening methods by switching from fluorescence activated cell sorting (FACS) to digital droplet PCR (ddPCR) screening as this is faster and results in less cell death. Additionally, we are working to improve and streamline our cryopreservation process. We are currently testing a high throughput freezing method in LN2 utilizing our Hamilton robot for cell banking.
How many lines is your team currently working on?
At any given time, our team is working on between 8 and 15 cell lines. Our gene-editing team currently consists of two scientists and four research associates.
What benefit do these lines offer researchers?
These cell lines go through a rigorous, three-part quality control (QC) workflow and are highly standardized. First, we screen 100-200 clones, and from that process we narrow it down to screen ~5-10 clones. We then collaborate with our assay development team to determine the final clone that will be released to the public along with a backup clone. These final two clones are then banked in up to 120 vials. The final clones are further screened through a trilineage assay, karyotype analysis for up to 30 metaphase cells, sterility, and mycoplasma testing. All the specific QC information is documented in our certificate of analysis for each line in our collection.
How often can researchers passage cells and maintain a normal karyotype?
We tested a handful of lines up to 10 passages and maintained a normal karyotype. We have not tested beyond 10 passages, so it’s highly recommended to thaw a new vial if you need to passage beyond 10. It’s also highly recommended, when you purchase a line, to create a working bank. Check out our protocol for cell line expansion and banking.
 |
Smith preserves cells in LN2. Photo by Erik Dinnel / Allen Institute |
What does the future of this collection look like? Are new lines continually added? Is there a methodology to what proteins are tagged?
We’ve tagged all the major structures so now we are moving on to more difficult structures and, because of this, we are facing new challenges. One cell line we are currently working on is CENP-A in the centromere (centralized in the chromosome). It’s very stressful on the cell to tag this particular structure. The Allen Institute for Cell Science regularly meets with experts in the field on our Advisory Council to decide which cell lines to develop.
Can you talk a little about your personal motivation for your work to develop the Allen Cell Collection?
It amazes me that we have created this whole collection with every major structure tagged. It’s so cool to see it all come together, and to see the amount we have accomplished. It’s also exciting to see more and more labs using our cell lines. The way that the Allen Cell Collection is supporting other labs really inspires me.
I personally hold really high standards and integrity for each cryovial that we produce, and I feel proud to know how much my teammates have contributed to this tremendous effort.